Manufacture of Steel
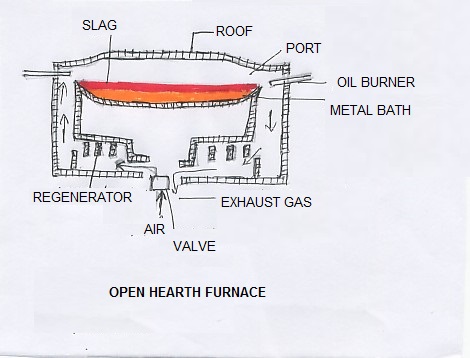
Open Hearth Process
Siemens and Martin of France developed the open hearth process. Initially the furnace was used for melting iron and steel scrap. Later on it was used for melting and refining of steel
scrap and pig iron. For the first time, both pig iron and scrap were charged to produce steel. The open hearth furnace is a shallow vessel lined with dolomite bricks. It is heated by
liquid and gaseous fuels using heat regeneration principle. The steel making temperature was about 1700'C. The charge is mixture of scrap and molten pig iron. The sponge iron forms part
of the charge. The scrap and sponge iron are initially heated to melting point. Then molten pig iron is poured into it from the blast furnace. To help in refining and slag formation some
amount of iron ore and limestone is added. Refining is carried out by blowing oxygen through lances. The basic slag is prepared by adding limestone and iron ore. The condition in the
furnace becomes oxidizing in nature.
In some cases, scrap and sponge iron are charged with partially molten iron. The impurities in the molten pig iron are diluted. The making of steel by using Bessemer converter and open hearth furnace is known as duplex process. In the first stage, the molten pig iron is partially refined in the Bessemer converter. Then it is further purified by blowing oxygen in the open hearth furnace. Towards the end of the process, when the impurity like silicon, manganese and calcium are brought down to the required level, the molten metal is tapped off through a tap hole in the furnace. Addition of ferromanganese and ferrosilicon are made to remove the impurities and oxygen present in the molten metal. Latter a small addition of aluminium is also made to deoxidize the metal. Carbon is removed through blowing of sufficient oxygen in the furnace.
In the acid process, lining of silica bricks is made in the hearth. Acid slag high in silica is produced on the metal during the refining process. Steel produced by the acid process is considered of very good quality. In the basic process, dolomite and magnesite bricks are used. In the basic open hearth process, the charging starts with iron ore, scrap, limestone, sponge iron and molten pig iron. The charge is heated and hot molten pig iron is poured into the furnace. The refining starts after charging is completely melted away. Then the oxygen is blown through the lances. Slowly, the basic slag forms above the molten metal. Then the slag is flushed out.
In the basic open earth process, the charging starts with iron ore, lime stone and scrap. The charge is heated and hot molten pig iron is poured in the hearth. The refining starts after charging is completed. Oxygen lancing was carried out. The basic slag was formed and slowly flushed out. After flushing is over, fresh lime stone is added to make high basic slag for removal of remaining amount of sulphur and phosphorus. As the generation of carbon monoxide increase, the fuel gas supply may be reduced. Oxygen is fed through the roof lance to accelerate the combustion. Blocking of heat is carried towards the end of the refining. Oxygen pressure is reduced to decrease the vigour of carbon oxidation. Ferromanganese and ferrosilicon are added to the molten metal for further blocking of heat. The fuel supply is also discontinued and liquid sample of steel is drawn for chemical analysis. De-oxidation of metal takes place due to reaction of manganese and silicon with oxygen. The manganese and silicon joins the slag when they are in excess quantity.
The slag is removed through a tap hole by tilting the furnace and molten steel is removed through the ladle. Nowadays tilting furnace are more in use than the fixed furnace. The open hearth furnace is smoothly operated by use of control systems. Measuring and control devices are provided for smooth function of the furnace. The open hearth furnace is also getting obsolete due to rigid system of its operation. So, new steel making processes are developed and adopted to reduce cost of production of steel and to produce high quality steel.