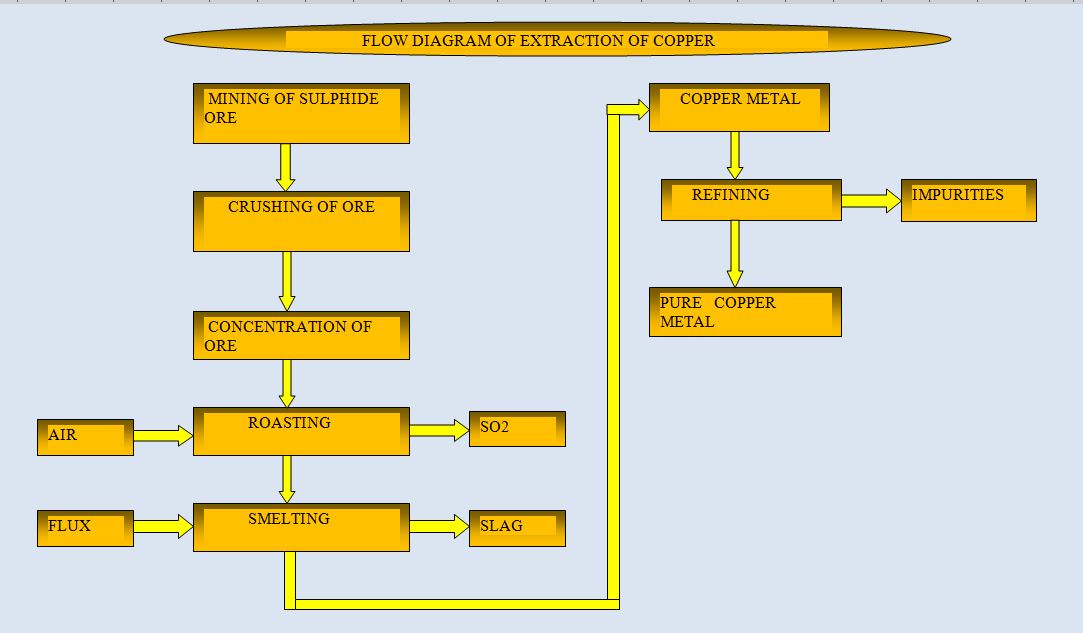
The process of extraction of copper depends upon the nature of the ore available. The important minerals used for extraction of copper are chalcopyrite and chalcocite.
The extraction of copper is mainly through the chemical reduction and the smelting process. In the first stage, the ore is crushed and concentrated by froth floatation process.
The concentrate is separated and dried. The concentrated ore is heated strongly in a current of air on the hearth of a reverberatory furnace. Air is supplied continuously to the furnace.
Sulphur is oxidized to SO2 and SO3 and separated into the atmosphere. The ore is broken into simple sulphides and partly oxidised.
2CuFeS2 + O2 -> Cu2S + 2FeS +SO2
2Cu2S + SO2 -> 2Cu2O + 2SO2
The roasted ore is mixed with coke and sand. These are charged into furnace that is waterjacketed from outside the furnace.
The chemical reactions which take place during smelting ore as follows:
2FeS + SO2 -> 2 FeO + 2SO2
Copper has a greater affinity for sulphur than oxygen. So,iron oxide is formed. The iron oxide is converted into slag by adding sand into the roasted ore.
Then the charge is heated in the furnace. a slag is formed and it is separated. The copper sulphide remained in the furnace. Then it was further heated and air is
continuously supplied to the furnace. The copper sulphide is reduced to molten copper and sulphur dioxide.
FeO+SiO2-> FeSiO3
Cu2S + O2-> 2Cu + SO2
The air supply to the furnace is stopped. The molten copper is separated into moulds. To obtain high purity copper, it is refined by electrolytic process.
In electrolytic refining, the copper received from the smelter is dipped into copper sulphate solution. A strip of pure copper is dipped into the solution to act as cathode.
The copper received from the smelter forms the anode and the pure copper strip forms the cathode. This electrolytic cell is operated and electrical current is switched on.
The impure copper dissolves in the copper sulphate solution. Pure copper is precipitated by electrolysis and deposited at the cathode end.
The impurities like iron and silicon settles down to the bottom of the cell. The pure copper is separated. The electrolysis is carried out between 40'C and 50'C.
The copper obtained from the refining process is found to be pure with 99.99% purity. Low grade ores of copper are used for extraction for copper and other metal.
The ore is crushed and cleaned in water. The clean ore is put in the water tank and water is added to it. After a prolonged period the sulphide ore is slowly converted
into copper sulphate and ferrous sulphate. The copper sulphate is separated and put into electrolytic cell. Copper is produced by electrolysis and separated for further refining