


| Symbol: | K |
| Melting point: | 63.38'C |
| Electron configuration: | [Ar]4s1 |
| Atomic number: | 19 |
| Atomic mass: | 39.0983±0.0001u |



| Ore | Common Name | Formula |
|---|---|---|
| Sulphate | Polyhalite | K2Ca2Mg [SO4],2H2O |
| Alunite | KAl2[SO4]2,[OH]6 | |
| Chloride | Sylvine | KCl |
| Carnallite | KMgCl3,6H2O | |
| Kainite | KCl, MgSO4,3H2O | |
| Nitrate | Nitre | KNO3 |
Potassium is a soft silvery-white alkali metal. Potassium does not occur in native state. A number of silicate minerals like feldspar, zeolite, muscovite and leucite are found containing potassium. But economic extraction of potassium and its salts is not possible from these silicates. So, economic extraction of potassium and its compounds is possible from some chlorides, sulphates and nitrates.
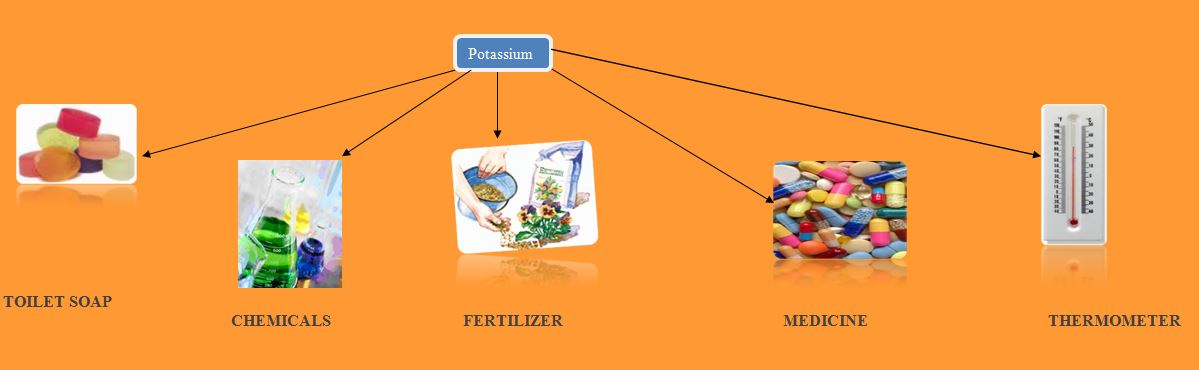
The most important use of potassium salt is as fertilizer. These salts are also used in glass and ceramic manufacturing. These salts are used in detergents.
Potassium is used in photovoltaic cells. It is also used in reagents for vegetable oil extraction. Potassium alloys are used in specified thermometers.
Potassium chloride is used as fertilizer. It is also used for base material for preparation of other potassium salts. Potassium hydroxide is used in the production of toilet soaps.
Potassium salts are also used in preparation of medicines and chemicals.
Potassium in pure form is not extracted due to the difficulty and cost involved in the process. Potassium salts like potassium hydroxide are produced for large scale uses. Sylivine is a combination of potassium chloride and sodium chloride. It is powdered and mixed with hot water. The powder is dissolved in the hot water to form a salt solution. The salt solution is separated for cooling. Crystals of potassium chloride forms and separates out. Potassium chloride is a white crystalline solid. It is separated and dried for further use Potassium hydroxide is prepared by electrolysis of solution of potassium chloride