


| Symbol: | Hg |
| Melting point: | -38.83'C |
| Electron configuration: | [Xe]4f145d106s2 |
| Atomic number: | 80 |
| Atomic mass: | 200.59±0.02u |



| Ore | Common Name | Formula |
|---|---|---|
| Sulphide | Cinnabar | Hgs |
Mercury is a liquid metal. It is also known as quick silver and liquid silver. Mercury was in use since 300 B.C. It is silvery white metal. It is usually found as sulphide. But it also exist in native form. Mercury forms amalgams with metals like silver. The source of mercury is the mineral cinnabar. It is a sulphide of mercury. The average yield of mercury is up to 10%. Cinnabar is found along with other sulphide ores. It is deposited from water of hot springs and volcanic eruptions. The important producers of mercury are Russia, USA, China, Spain, Algeria, Turkey, Finland, and Yugoslavia.
Mercury is mostly used in drugs and chemicals. Mercury is also used in extraction of silver and gold. It is used in manufacture of caustic soda. It is also used in instruments like thermometers, pressure regulators, manometers and automatic electric switches. Mercury is used in mercury vapor lamps and fluorescent tubes. It is used in dental surgery operations. Mercury is used in chemicals and paints. Mercury oxide is used as pigment in oil paints. It is also used in antiseptic ointments. Mercury chloride is used as calomel electrodes which are used in electrochemical works. It is used in preservation of timber. It is also used in sterilizing hand and surgical instruments. Mercury sulphide is used in as pigment. Mercuric sulphate is used for preparation of mercury salt.
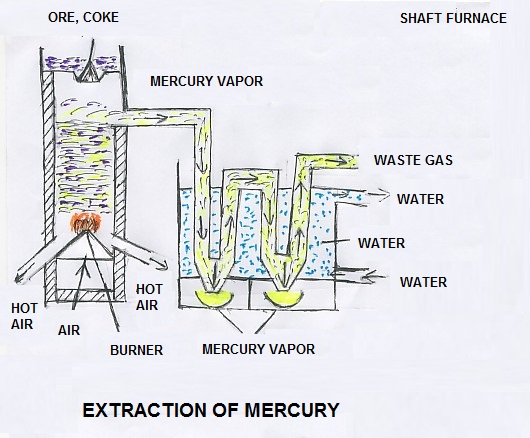
At the initial stage, the ore is concentrated by froth floatation process. The ore concentrate is mixed with coke and charged into a shaft furnace. The coke is fired and the ore is roasted. The sulphur gas combines with oxygen, forms sulphur dioxide and releases to the atmosphere. The mercury oxide is formed by combination of mercury and oxygen. Due to reduction, the metal is separated and oxygen is removed. As carbon dioxide and carbon monoxide is passed through it, the mercury is converted into vapor and condensed in to its natural stage. It is refined to produce pure metal of mercury. At the refining stage the metals like zinc, lead and silver are separated for further use.