


| Symbol: | Co |
| Melting point: | 1495'C |
| Electron configuration: | [Ar]4s23d7 |
| Atomic number: | 27 |
| Atomic mass: | 58.933195±0.000005u |



| Ore | Common Name | Formula |
|---|---|---|
| Arsenide | Smaltite | (Co,Ni)As3-n |
| Sulphide | Linnaeite | Co3S4 |
| Sulpharsenide | Cobaltite | (CoAsS) |
| Hydrated Arsenate | Erythrite | Co3(AsO4)2,8H2O |
| Oxide | Asbolite | Co2O3,Fe2o3,Mn2O3 |
Cobalt is very close to nickel in appearance and properties. It has high melting point of 1500'C. It is malleable and resistant to corrosion. It is used for producing ferro alloys and high speed steel. It is also used in non-ferrous alloys. It is used for electroplating of metal like steel. The compounds of cobalt have application in medical industries and chemical industries. Cobalt mineral deposits are found near pyrrhotite deposits. It is also found in association with nickel, arsenic, silver and copper. The minerals of cobalt are found in association with basic and ultra-basic rocks. The important minerals of cobalt are smaltite, cobaltite and asbolite.Important producers of cobalt are Zaire, Zambia, Canada, Finland, Russia, Cuba, China, France and Finland.
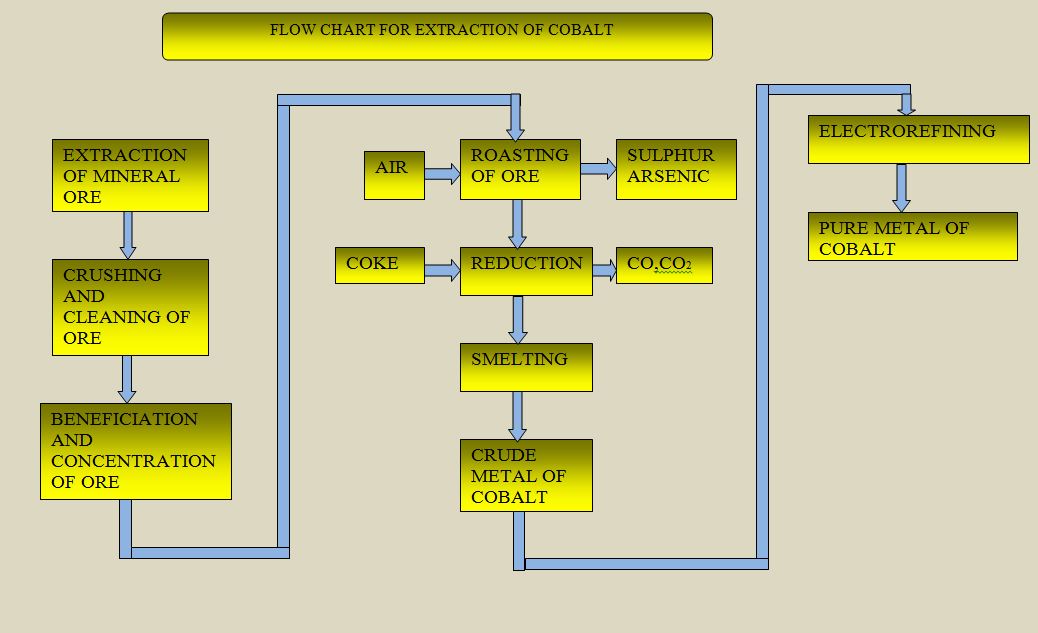
Cobalt is second only to iron in terms of its strong ferromagnetic properties. Therefore, it finds use in permanent magnet. Cobalt is an important constituent of super high speed steel. It is used as the binding matrix of cemented carbide tool materials. The corrosion resistance of cobalt is very high. At high temperature, its resistance increases. Most of the cobalt alloys are very strong. These alloys are used in gas turbines and jet engine blades. Cobalt alloys are also used in orthopaedic implants. Cobalt is an essential constituent of marging steel. It contains iron, nickel, cobalt, molybdenum, titanium, alluminium and carbon. Cobalt steel alloys are used in space-crafts, air-crafts, ships and auto-mobiles. Some amount of cobalt in stainless steel helps in checking the rusts and improves the appearance of the alloys.
Most of the cobalt alloys are shaped by investment casting. These castings are very strong. Such alloys are used in gas turbine vanes and jet engines blades. Cobalt based alloys are also used in orthopaedic implants. A popular alloy containing 65% cobalt, 25% cobalt, 6% molybdenum,1% nickel and remaining amount of iron makes a very strong alloy for use in instruments used under stress conditions. Another alloy contains 20% cobalt, 10% nickel, 0.1% carbon and remaining portion as cobalt. It is used in production of specialized surgical equipments. Cobalt is an essential constituent of Marging steel. It contains 18% nickel, 12% cobalt, 5% molybdenum and small amount of titanium, aluminium and carbon. The remaining portion is iron.