| Symbol: | Sn |
| Melting point: | 231.93°C |
| Electron configuration: | [Kr]4d105s25p2 |
| Atomic number: | 50 |
| Atomic mass: | 118.71 u |


| Ore | Common Name | Formula |
|---|---|---|
| Oxide | Cassiterite or tinstone | SnO2 |
| Sulphide | Stannine or tin pyrite | Cu2SnFes4 |
Tin is a bright white metal. It is soft, malleable and ductile. Tin reacts softly with dilute Hydrochloric acid. Tin is resistant to corrosion. It melts at 232 °C. It exists in three allotropic form in grey, white and rhombic. Tin can be rolled into thin sheets. The important use of tin is in the manufacture of tin plate. Besides that tin is used in production of alloys like bronze type metal, gun metal, bell metal, fusible metal and bearing metals. Tin alloys and tin metal are used in foils for wrapping articles. The major tin ore producing countries are Malaysia, Indonesia, Myanmar, Cambodia, Laos, Brazil, Bolivia, Thailand, Australia, Zaire and Nigeria. The tins smelting countries are Malaysia, Indonesia, Thailand, Bolivia, Russia, China, UK, Brazil, Australia, USA and India.
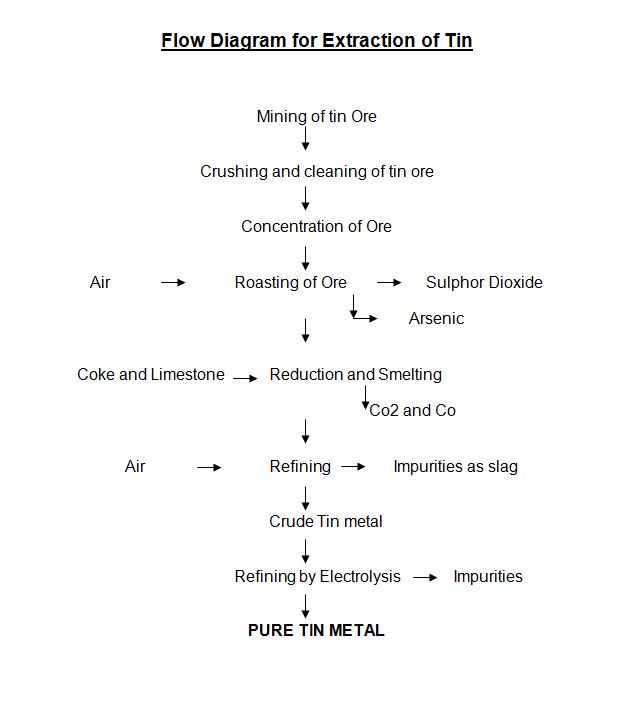
The tin ore cassiterite is crushed and cleaned properly. The ore is concentrated by froth floatation process. The ore concentrate is roasted in a reverberatory furnace. Air is supplied into the furnace. Due to heating of the ore, sulphur, arsenic and other impurities are removed. The roasted ore is mixed with coke and reheated in the same furnace. The tin oxide is reduced to tin and oxygen is removed as carbon dioxide and carbon monoxide. Small amount of lime stone powder is added to the ore concentrate as flux. Silicon combines with calcium to form slag. The metallic concentrate is shifted to a besemer converter. Oxygen is blown through the molten metal. The impurities are converted into slag. The molten tin metal is removed for further use. The impurities are removed from the furnace.
For making alloys of tin pure tin metal is required. To get high purity of tin metal, electro refining of tin metal is carried out. The electrolyte commonly used in fluosilic acid with sulphuric acid and tin is dissolved in it. Some times sodium thiostannate and sodium sulphide are used as electrolyte. The anode is crude tin metal and cathodes is copper plate. Sometimes lead is also used as cathode.
Electroplating of tin is made for providing a coating to iron and steel for corrosion resistance and good appearance. The electroplating may be done hot dipping and chemical replacement. Plating baths are both acidic and alkaline in nature. Sodium stannate and potassium stannate are used of preparation of bath. Electro deposition is carried out by electrolysis of tin solution. Chemical replacement is carried out by dipping of steel plates in solutions where tin is dissolved. Tin replaces some iron and get deposited on the surface of the steel plate
In case of electroplating the part of the metal which is to be electroplated is formed as cathode. This part is dipped into a solution of tin to be plated by passing direct current before the cathode part and another electrode. The thickness and brightness of the coat depends upon temperature, current density, time and composition of bath. Hard plating rights corrosion better and become popular. It gives thin and uniform coating. The tin metal powder is fed through a melting flame to blow finely divided liquid particles on to the surface, ships, bridges, harbour cranes and other under water metallic equipments are sprayed by flame. It produces thick coat. Under water pipes are coated by hot dipping in tin metal bath. Electroplated by tin metal, the steel metal sheets are used for packing of foods as container. The tin plated steel sheets are mechanically isolated from the atmospheric and water related corrosive activities.
Tin based bearing metals contain tin, antimony and copper. This alloy can take high pressure and load. It provides a minimum of friction when the bearings are in contact with shaft. Babbit metal is a tin based white metal. It contains 88 percent tin, 8 percent antimony and 4 percent copper. It is used bearings with cast iron boxes. It can work as bearing in high pressure and load condition. The babbit metal is used in automobile and wire raft. Solders are alloys of lead with tin, bismuth and cadmium. It is used in electrical instruments. Fusible alloys are alloys of Bismuth, lead, tin and cadmium. This is used for electrical fuses, sprinkler system and boiler plugs. Type metals are alloys of Antimony, tin and lead. It is used in printing industries.