

| Symbol: | Pb |
| Melting point: | 327.5'C |
| Electron configuration: | [Xe]4f145d106s26p2 |
| Atomic number: | 82 |
| Atomic mass: | 207.2±0.1u |



| Ore | Common Name | Formula |
|---|---|---|
| Sulphide | Galena | PbS |
| Sulphate | Anglesite | PbSO4 |
| Basic Sulphates | Plumbojarosite | PbFe6(OH)2(SO4)4 |
| Chlorophosphate | Pyromorphite | 3Pb3P2O8,PbCl2 |
| Chloroarsenate | Mimetite | 3Pb3As2O8PbCl2 |
| Chlorovanadate | Vanadinite | 3Pb3V2O8PbCl2 |
| Chromate | Crocosite | PbCrO4 |
| Molybdate | Wulfenite | PbMoO4 |
| Carbonate | Cerussite | PbCO3 |
Lead is the heaviest metal in the list of common metals. It is very soft, malleable and ductile. It can be rolled easily. Lead is resistant to corrosion. Many acids have no chemical actions on lead. Because of this character, it is used for water pipes, roof coverings, cable coverings, chemicals and paints. Lead melts at 327'C. Lead is rarely found in native state. Its color is bluish grey. The freshly cut surface shows bright metallic lustre. The surface can be scratched easily. Lead is soluble in nitric acid. Major lead producers of the world are U.S.A, Japan, Russia, Germany, U.K, Canada, Australia, France and India. The countries like Australia, U.S.A, Russia, Canada, Peru, Mexico, South Africa, Morocco, India and Yugoslavia are leading producer lead minerals. The primary ore mineral of lead is Galena. Other important ores are Cerussite and Anglesite. The elements like Zinc and Antimony are associated with lead in ore deposits.
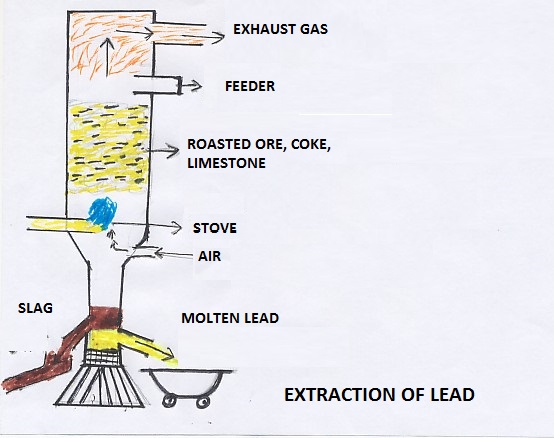
Galena is the most important ore of lead. It contains lead up to 8%. It is always associated with Sphalerite, that is an ore of zinc. The ore of lead is crushed and cleaned by washing. The cleaned ore is concentrated by froath-floatation process. The concentrated ore is separated and dried properly. The ore concentrate is roasted in a reverberatory furnace at moderate temperature. Air is supplied into the furnace during the roasting.
After the roasting is over, air supply is stopped. Some amount of Galena concentrate is added to the roasted ore. The temperature is increased and the air supply starts. Lead sulphide reacts with Lead Oxide and Lead Sulphate to produce lead metal.
The molten metal is collected at the bottom of the furnace. It is separated into ladles and converted into metal castings.