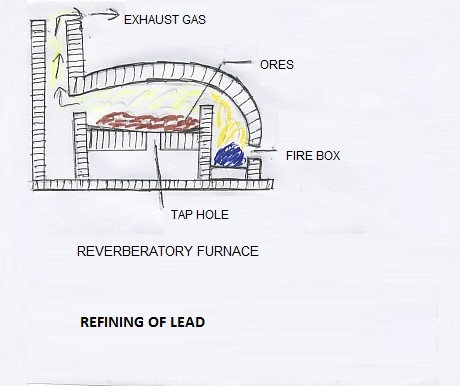

The lead metal extracted through the blast furnace process contains impurities like silver, copper, tin, iron, bismuth and antimony. The lead has low melting point and it can be separated from impurities with high melting point. The molten lead is removed to a furnace where blowing of air is carried out through the molten metal. So, the impurities come to the surface as a slag and are separated from the molten lead. The impurities can also be removed by a process when the metal is remelted in a reverberatory furnace. Air is supplied to the furnace. The impurities get oxidized and come out in the form of a slag. The slag is separated and molten metal is taken out for further use. Due to the cost of silver, it is separated specially by desilverisation process and used separately. But nowadays lead is also refined by electrolytic process. In this process the impurities like bismuth and antimony can be easily separated. The electrolyte used in this process is fluosilisic acid.
The anodes are formed by crude lead metal which are cast with logs to separate them and have electrical contacts with bush bars. The cathodes are made up of electrolytic copper. When the process starts, the power is switched on. Slowly, the lead is dissolved into the electrolyte. During the electrolysis, the pure lead is redeposited at the cathode point. After completion of the process, the lead metal is separated from the cathode end. It is cleaned properly and dried for further uses. A new electrolyte called sulphamic acid is also used in the electrolytic refining of lead.



Lead is a soft and malleable metal. It has high density, low melting point, low strength and low electrical conductivity. It has coefficient of thermal expansion. It has corrosion resistance and good lubricating character. It can absorb gamma rays and x-rays. Lead can be hot worked at room temperature. Its recrystallization temperature is below room temperature. Lead has a number of applications.
It is used in storage batteries. It is also used as anti knocking in petroleum products. But such use is discontinued to check lead pollution from the exhaust gases of auto-mobiles. It is also used as shield material for x-rays and gamma rays. It is used in lubricants, chemicals and paints. Lead is alloyed with copper, zinc and antimony to produce alloys. Solders are alloys of lead used in soldering works. Soft solders are alloys of lead , tin, bismuth and cadmium. Hard solders are alloy of silvers , copper and zinc. These are also called copper solders. The fusible alloys are formed by alloying of lead , bismuth, tin and cadmium. These alloys are used in electric fuses , sprinkler systems, boiler plugs, pressure cooker plugs and fire safety devices. Type metals are used in printing industry. These are alloys of lead, tin and antimony. Type metals are used in printing industries. These are alloys of lead, tin and antimony. Antimony increases the hardness and wear resistance. Tin increases fluidity ,fineness of structure and reduces brittleness. Babbit metal of lead is produced by alloying of lead , tin , copper and antimony. This metal is used in aircraft and auto-mobile industries. Bearing metals are also produced by lead alloys. Alloyed with small amount of arsenic, lead is used to produce shots for ammunition. Bearing metals contain lead,tin and antimony. Gun metal is produced by alloying of lead,zinc,tin and copper. Gun metal is used for making gears and bearings. Peweter is produced by alloying of lead and tin. It is used for making utensils. Special lead alloys are produced to make lead chamber for manufacturing sulphuric acids.