Extraction of Zinc
Zinc is extracted by reduction method and electrolytic method. The zinc ore is crushed and cleaned properly. The ore is concentrated by froth-floatation process.
The zinc sulphide ore is roasted in a reverberatory furnace. Air is supplied to the furnace the zinc sulphide is converted to zinc oxide.
2 ZnS + 3O2-> 2ZnO + 2SO2
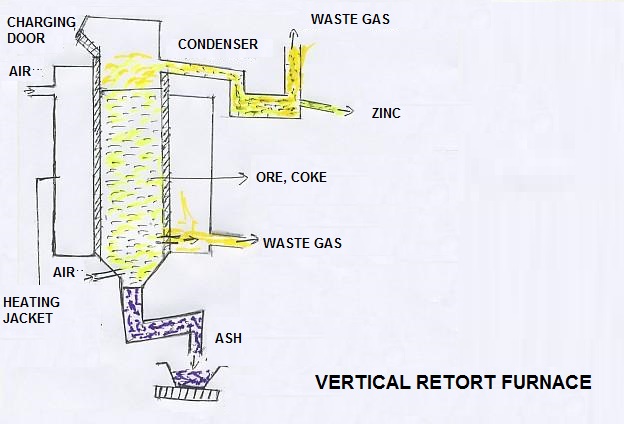
The sulphur dioxide is formed as a gas and reused in the manufacture of the sulphuric acid. The zinc oxide and coke is mixed and put in a vertical retort.
The coke is fired in the retort . Air blast and producer gas is put inside the coke bed and zinc oxide. After sometime, the oxygen is removed and zinc oxide is reduced into zinc.
Carbon dioxide and carbon monoxide are formed and move out as waste gases. When further heat is applied, the zinc becomes volatile and moves out of the retort.
It Is condensed in a condenser. On cooling, it is solidified and purred zinc metal is prepared by pouring molten metal into cold water.
When zinc concentration is low in the ore , the electrolytic process is adopted for zinc and other associated materials. The process is to bring the zinc from the ore into solution as zinc sulphate and then electrolyzing it with insoluble anode. The electrolyte used is sulphuric acid. The roasted ore concentrate is leached with sulphuric acid. The solution is purified and impurities are removed. When the roasted ore is treated with sulphuric acid , zinc dissolves in the acid. The solution containing zinc and sulphuric acid is taken out for electrolysis. The electrolytic cell is constructed by reinforced concrete materials. The cells are arranged in stepwise position. Electrolyte flows by gravitation from the top cell to the bottom cell. The anodes used in this process are made up of lead and cathodes are made of aluminium. The solution is put on the top cell and electrolysis starts. After sometime, the zinc metal is slowly deposited at the cathode point. The solution is removed to the second cell and some amount of sulphuric acid is added to the cell. Undissolved roasted concentrate may be put into it. The electrolyte may be properly circulated. Electrolysis process starts and it is found that zinc is deposited into the cathode point. , the process may be repeated for other cells so that the roasted ore may be completely dissolved in the sulphuric acid solution. The process of electrolysis may be continued for optimum extraction of zinc metal. The zinc metal deposited on the cathode point is removed and cleaned properly. The cleaned zinc metal is dried and taken for specific uses. The remaining solution may be kept for further use and the impurities may be removed from the cell.